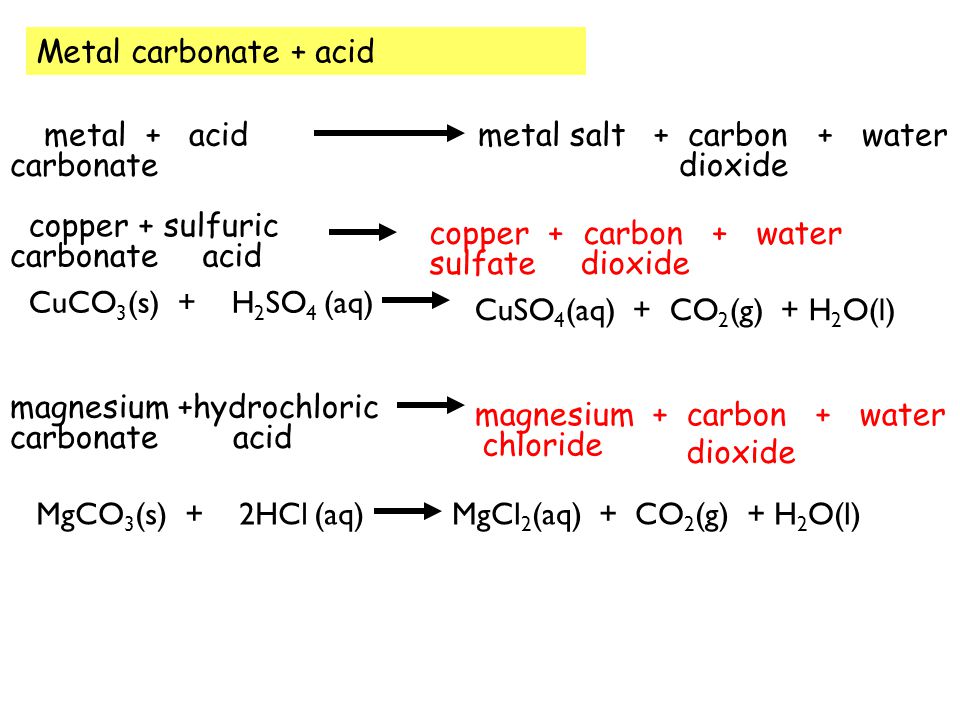
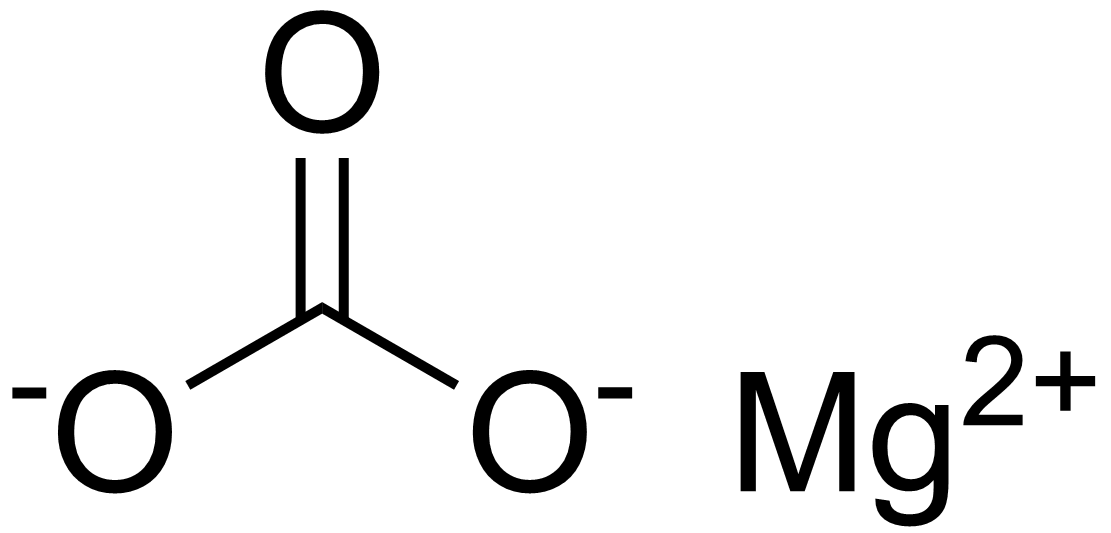
SOLVED:Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction that occurs
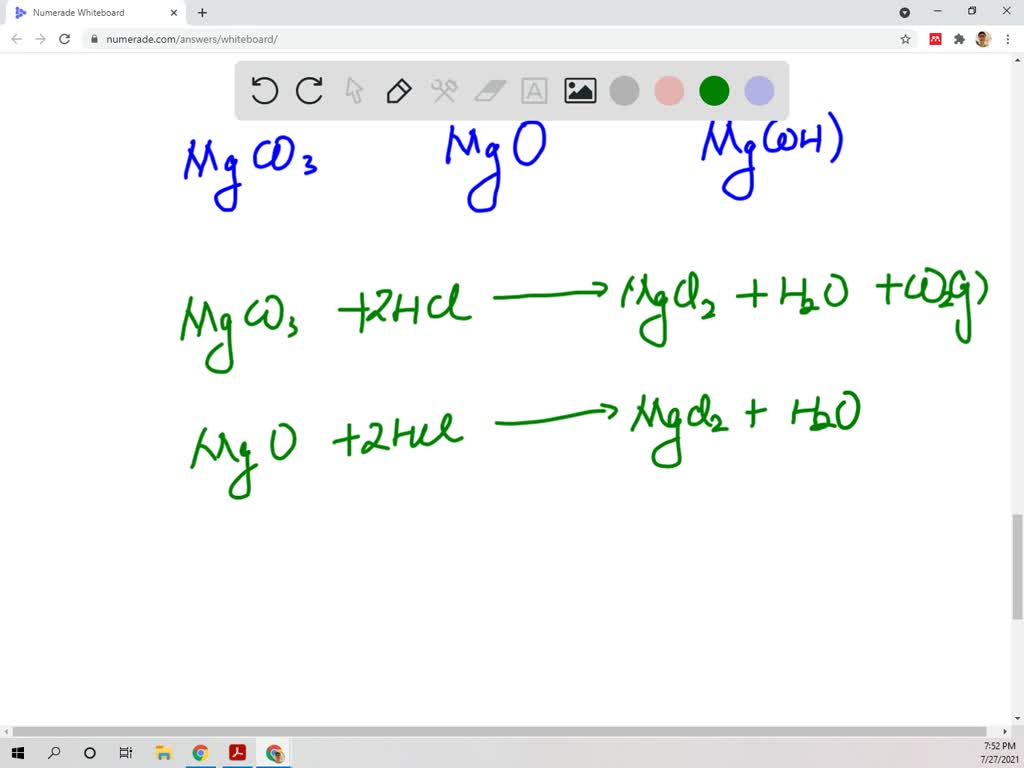
SOLVED: Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a netionic equation for the reaction that occurs
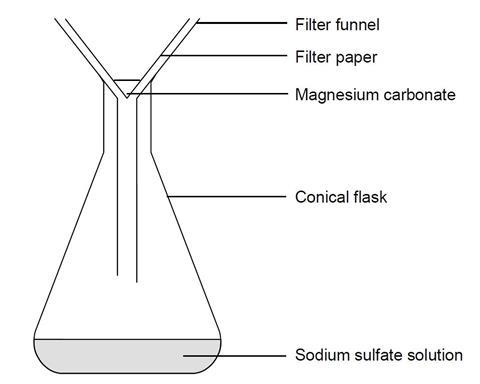
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education

5 g of a sample of magnesium carbonate on treatment with excess of dilute hydrochloric acid gave 1.12 L of CO(2) at STP . The percentage of magnesium carbonate in the mixture is

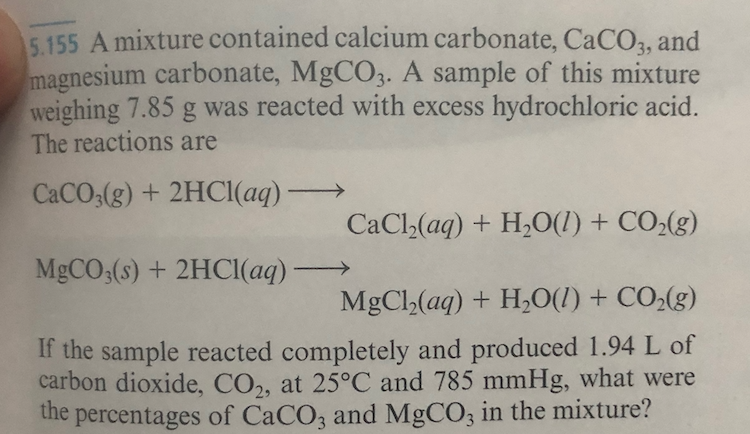
SOLVED:A 6.53 -g sample of a mixture of magnesium carbonate and calcium carbonate is treated with excess hydrochloric acid. The resulting reaction produces 1.72 L of carbon dioxide gas at 28^∘ C

SOLVED:The mineral dolomite contains magnesium carbonate. This reacts with hydrochloric acid. MgCO3(s)+2 HCl(aq) →CO2(g)+MgCl2(aq)+H2 O(ℓ) (a) Write the net ionic equation for this reaction and identify the spectator ions. (b) What type

SOLVED: The active ingredients in a tablet of a popular antacid are listed as 585 mg of CaCO3 and 112 mg of Mg(OH): These each react with hydrochloric acid found in your

Magnesium carbonate reacts with hydrochloric acid to form magnesium chloride, carbon dioxide and water Translate and balance the equation - Science - Chemical Reactions and Equations - 12554199 | Meritnation.com

For Your Research. The Four Research Questions 1.What is the chemistry (including an equation) of the process? 2.What are the factors that impact on the. - ppt download