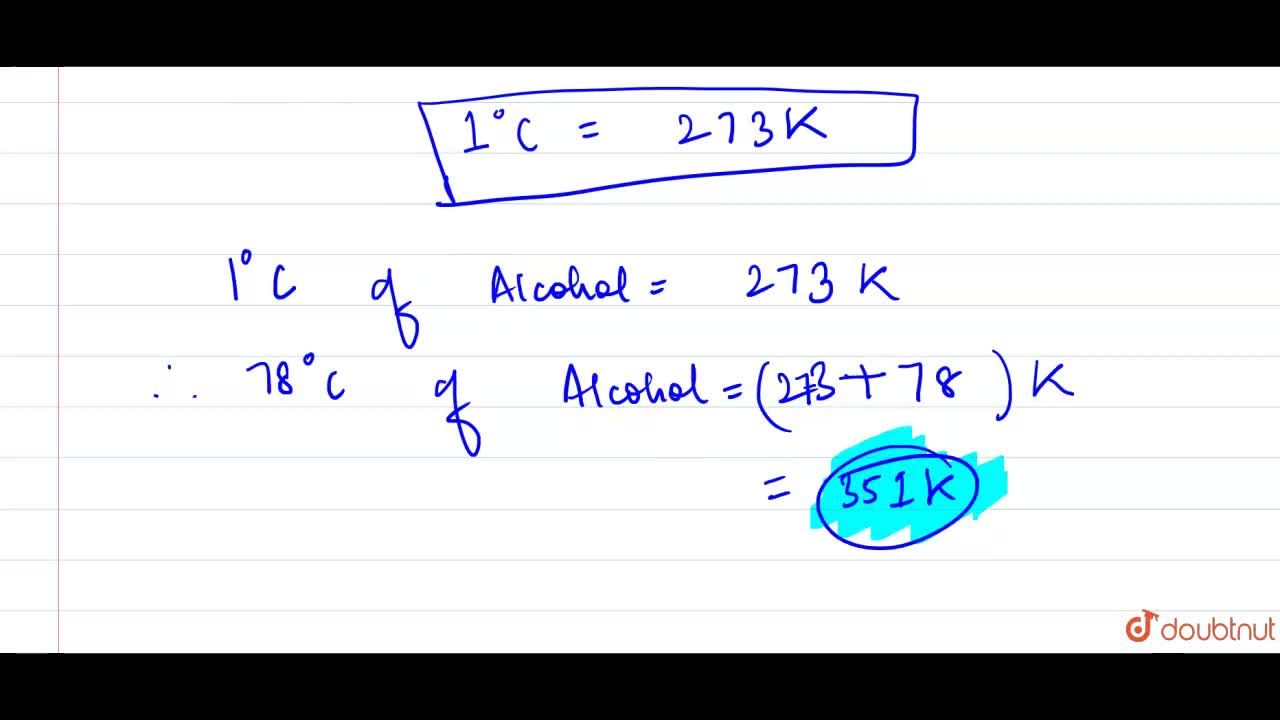
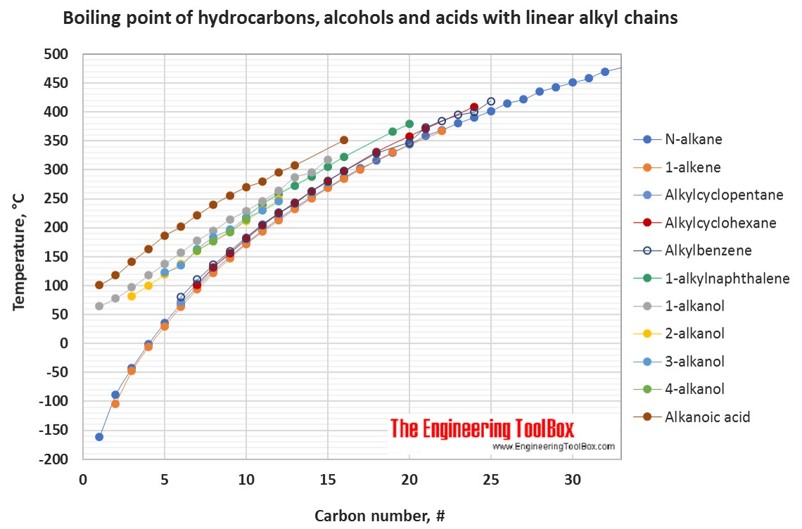
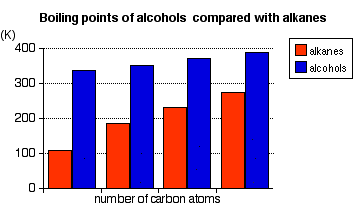
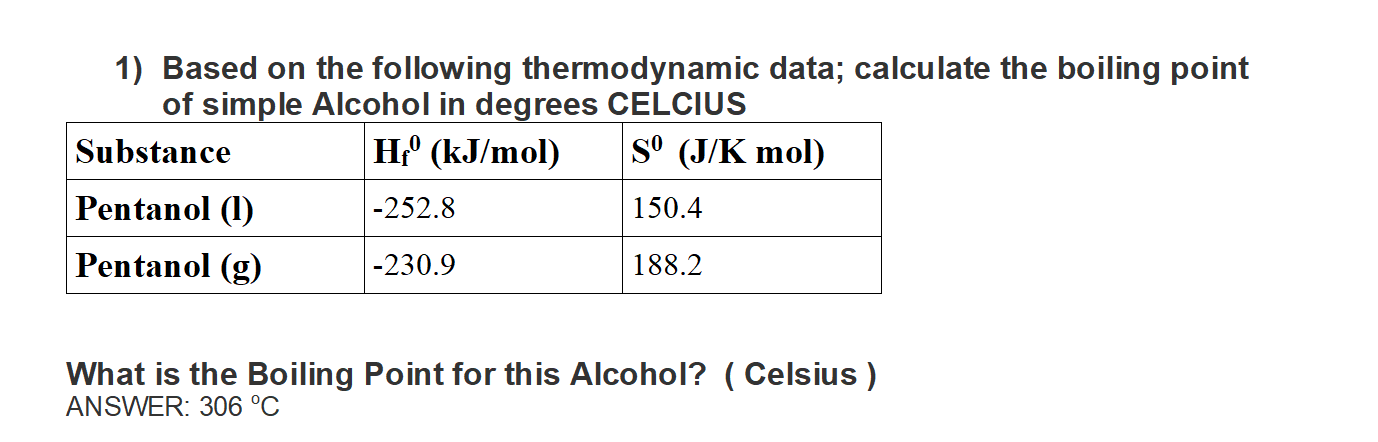
SOLVED: Ethanol, commonly known as ethyl alcohol, has a boiling point of 351 K. Convert this temperature to degrees Fahrenheit and degrees celsius.
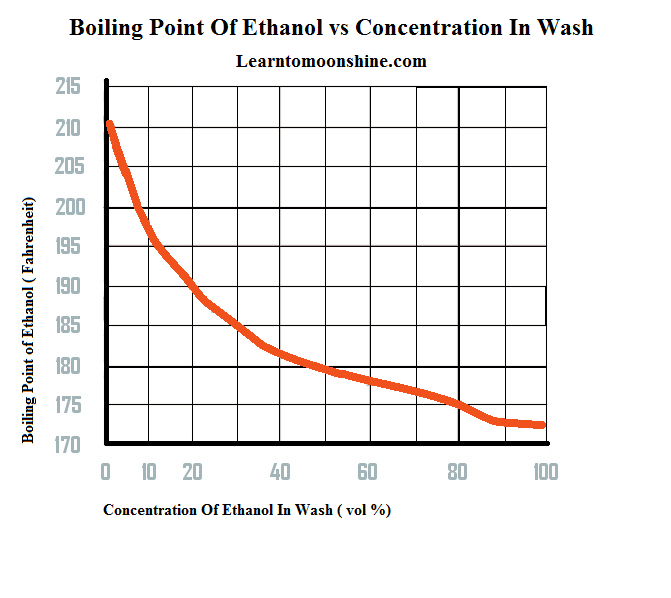
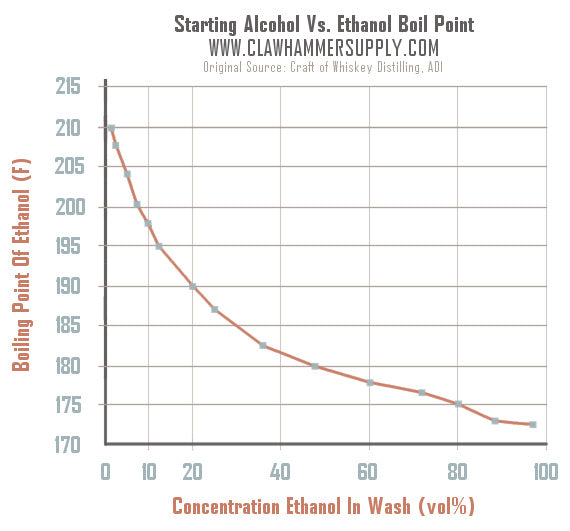
Ethanol has a boiling point of 78°C and water has a boiled point of 100°C. How can distillation be used to separate a mixture of water and ethanol? - Quora
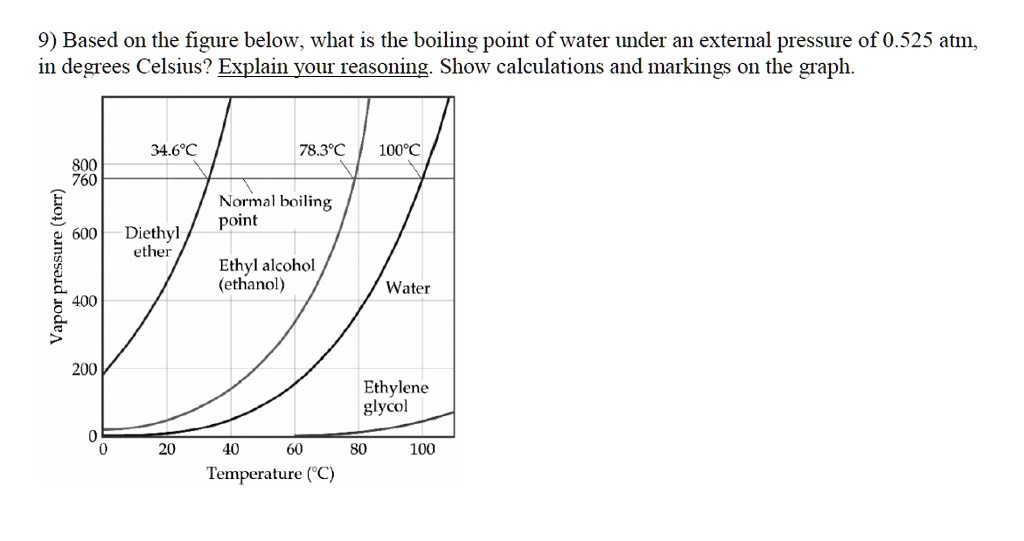
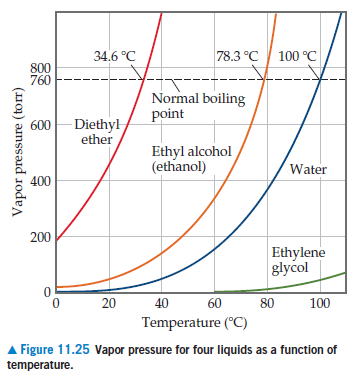
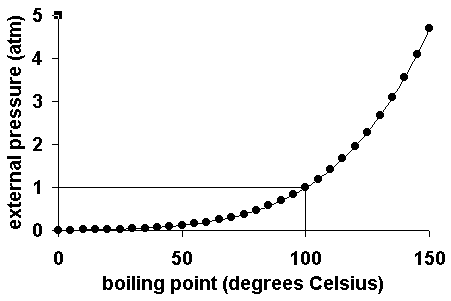
SOLVED: 9) Based on the figure below; what is the boiling point of water under an external pressure of 0.525 atmn; in degrees Celsius? Explain yourreasoning: Show calculations and markings 0n the









:max_bytes(150000):strip_icc()/whiskey-distillation-157532646-57a236655f9b589aa91b8ff0.jpg)