![SOLVED: 6. Calculate the total hardness, calcium hardness and magnesium hardness for a water sample having a pH of 7.2 and the following concentrations of ions: [Ca ] = 40 mgL [Mg ] = SOLVED: 6. Calculate the total hardness, calcium hardness and magnesium hardness for a water sample having a pH of 7.2 and the following concentrations of ions: [Ca ] = 40 mgL [Mg ] =](https://cdn.numerade.com/ask_previews/32bca3e4-ee47-44d7-8fb8-7e86804add2d_large.jpg)
SOLVED: 6. Calculate the total hardness, calcium hardness and magnesium hardness for a water sample having a pH of 7.2 and the following concentrations of ions: [Ca ] = 40 mgL [Mg ] =

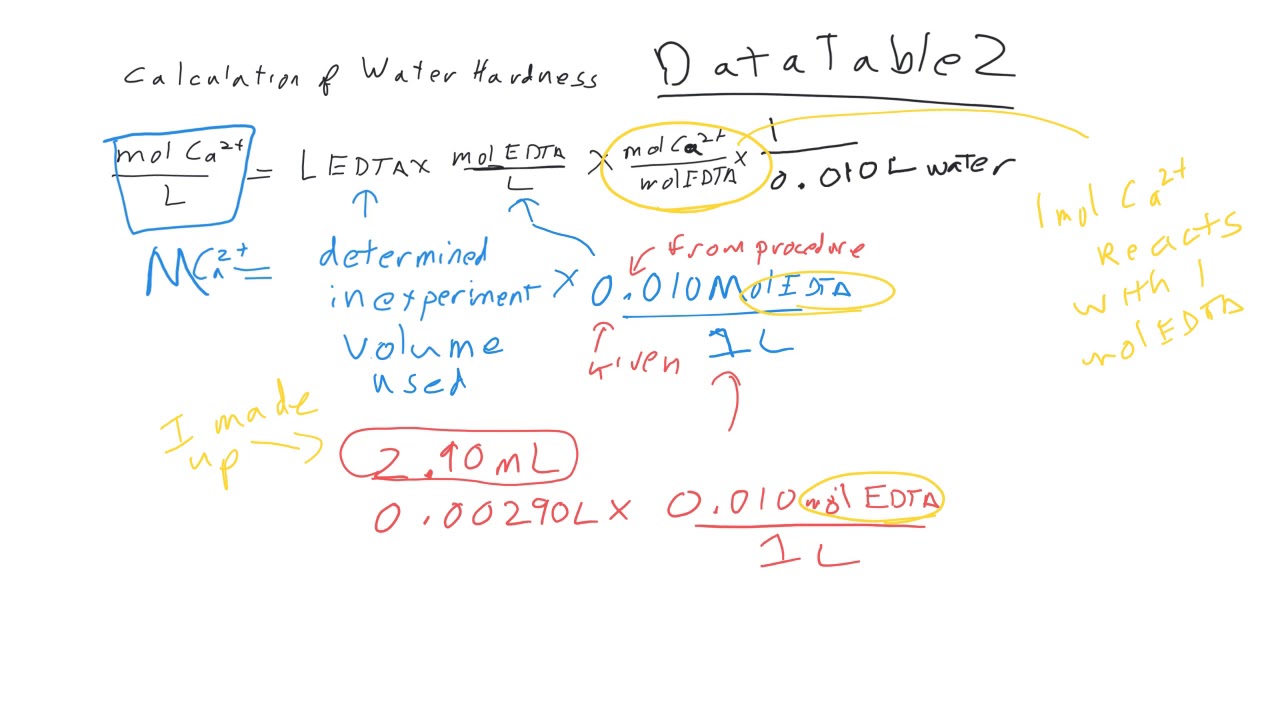
SOLVED: on 1i (These are post-lab calculations from the Water HardnessLab) Calculate the concentration of calcium in the calcium standard (inunitsppm Ca)used for the Water Hardness Lab and calculate the ppm calcium

One litre of sample of hard water contain 4.44mg CaCI2 and 1.9mg of MgCI2. What is the total hardness in terms of ppm of CaCO3 ?

SOLVED: Problems Calculate total hardness, carbonate hardness, and non-carbonate hardness of the samples What is the degree ofhardness of the sample (sof, moderate; hard, very hard)? Why might we necd to know

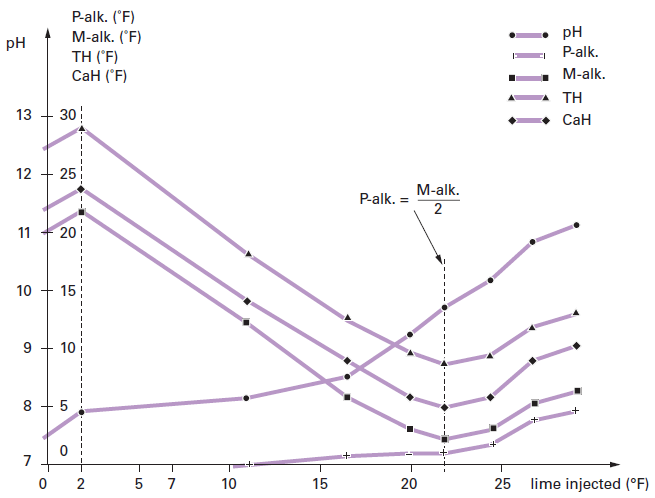
21 Water Softening Calculating Calcium H - 211 21 Water Softening TOPICS Water Hardness Calculating - Studocu

Concentrations of dissolved calcium and magnesium in soft and hard water | Download Scientific Diagram

Hardness CE Lab. Definition Hardness of water is a measure of its capacity to precipitate soap and is caused mainly by the presence of divalent. - ppt download

Naturally Occurring Elements in Nebraska's Groundwater: Part 1 of a Series - Calcium and Magnesium | UNL Water