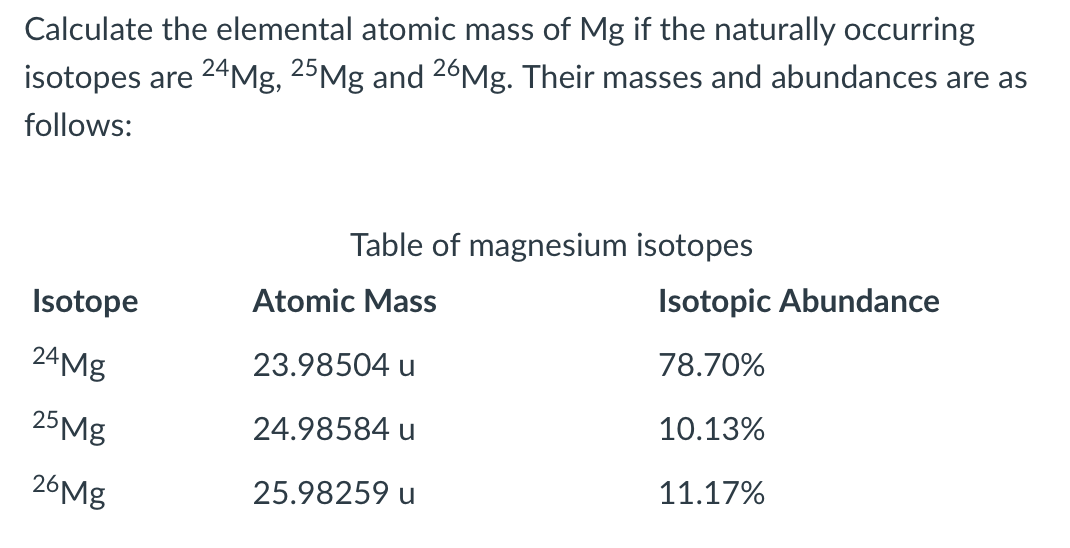
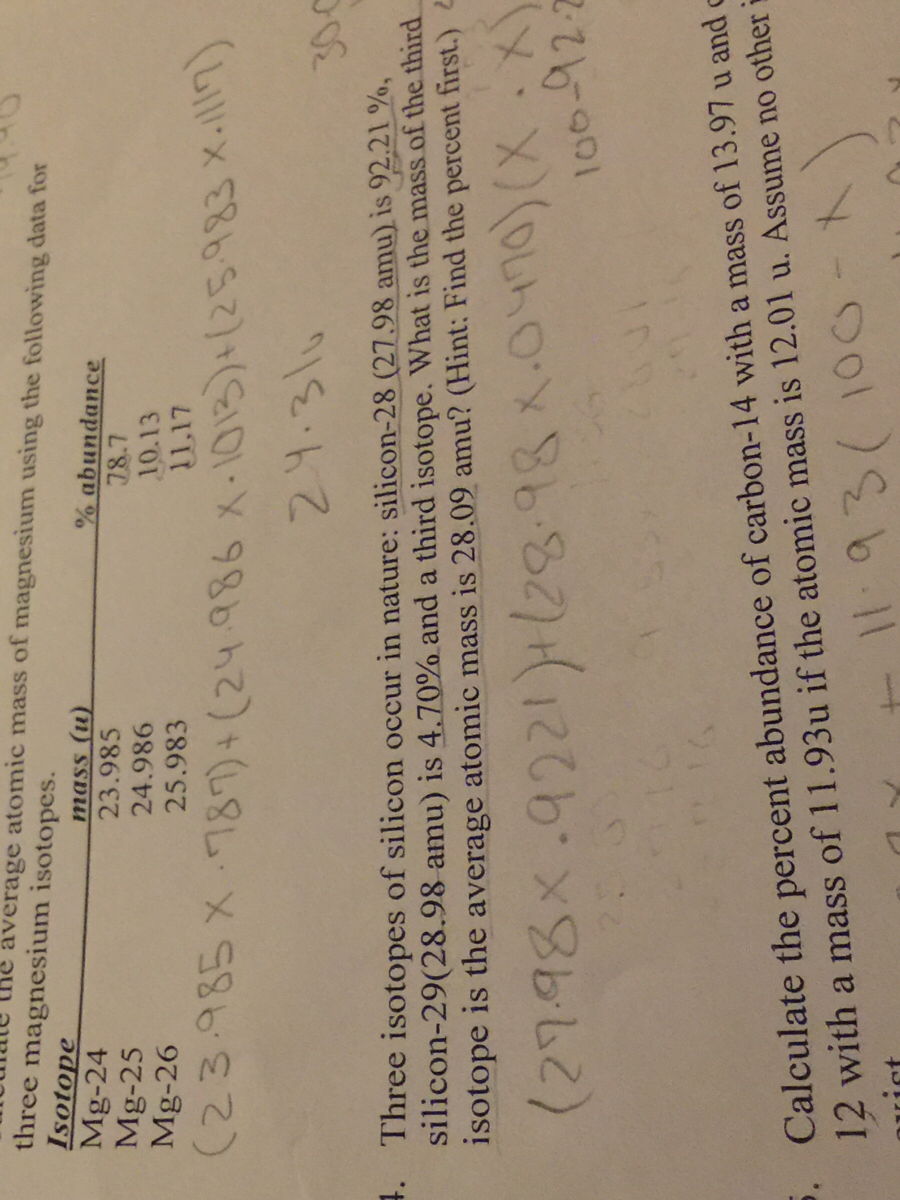1 Warm Up Isotopes Mass of Isotope Abundance 24 Mg =24.0 amu 78.70% 25 Mg = 25.0 amu 10.13% 26 Mg = 26.0 amu 11.17% Calculate the mass average of magnesium. - ppt download

Lick index diagram to diagnose the magnesium-to-iron abundance ratio in... | Download Scientific Diagram
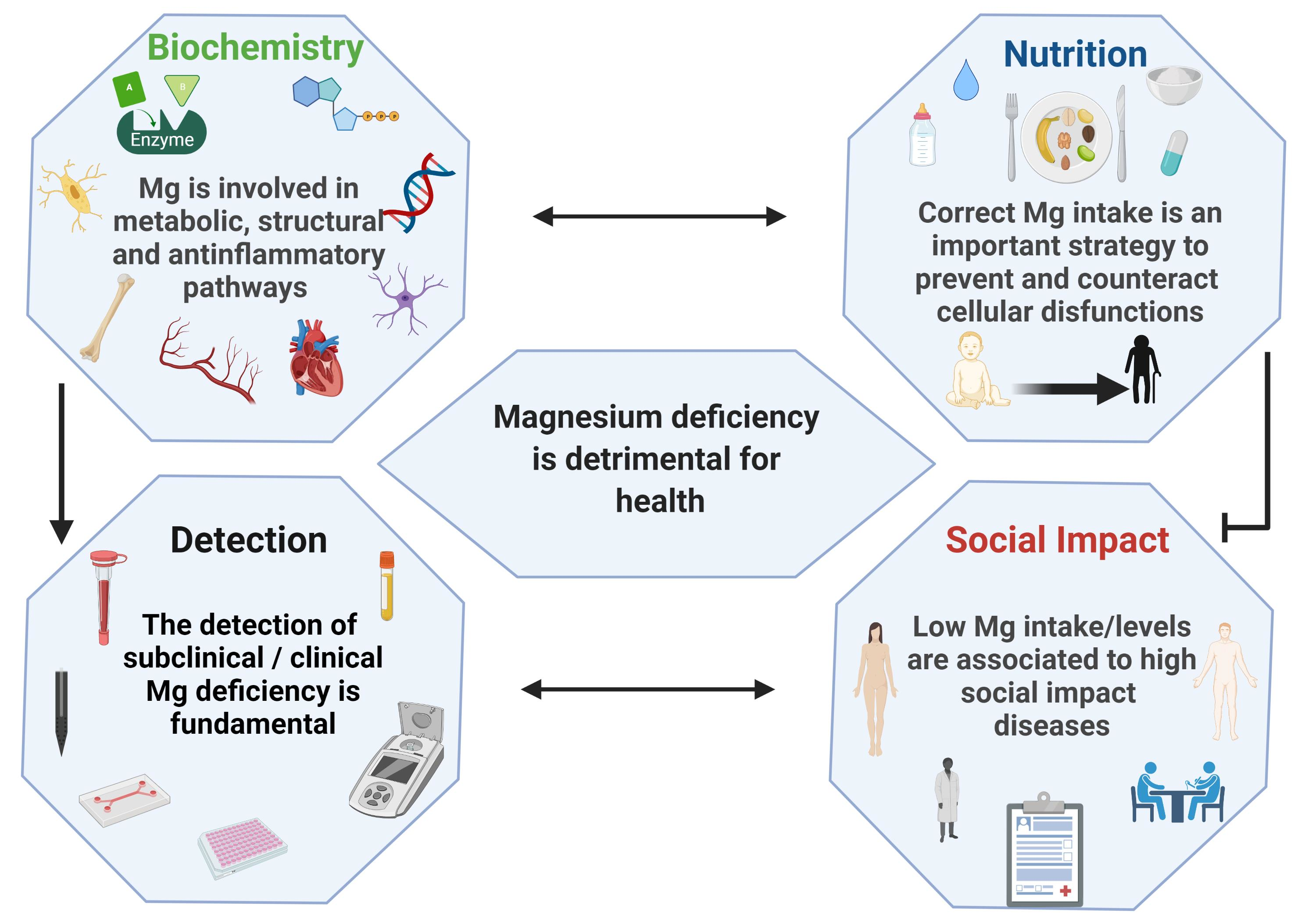
Nutrients | Free Full-Text | Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency

Surface atomic magnesium abundance of magnesium strips after soaking in... | Download Scientific Diagram
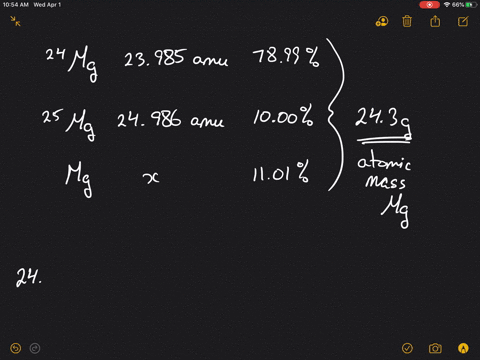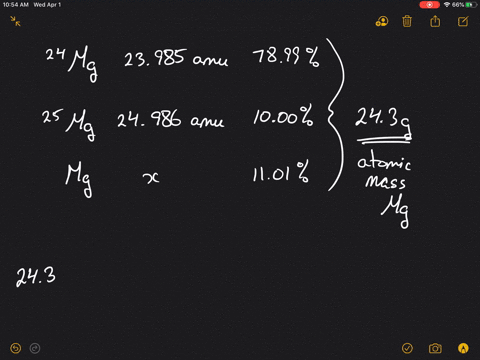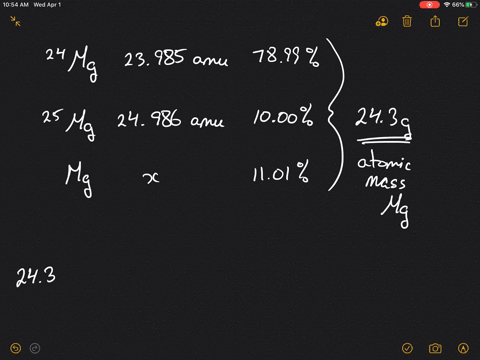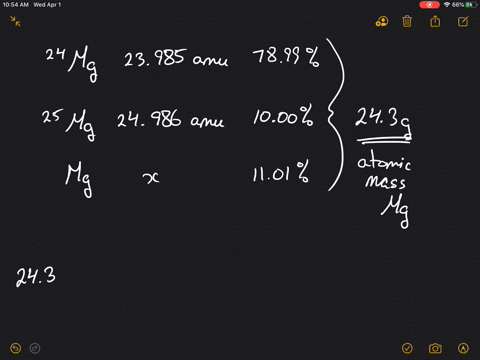
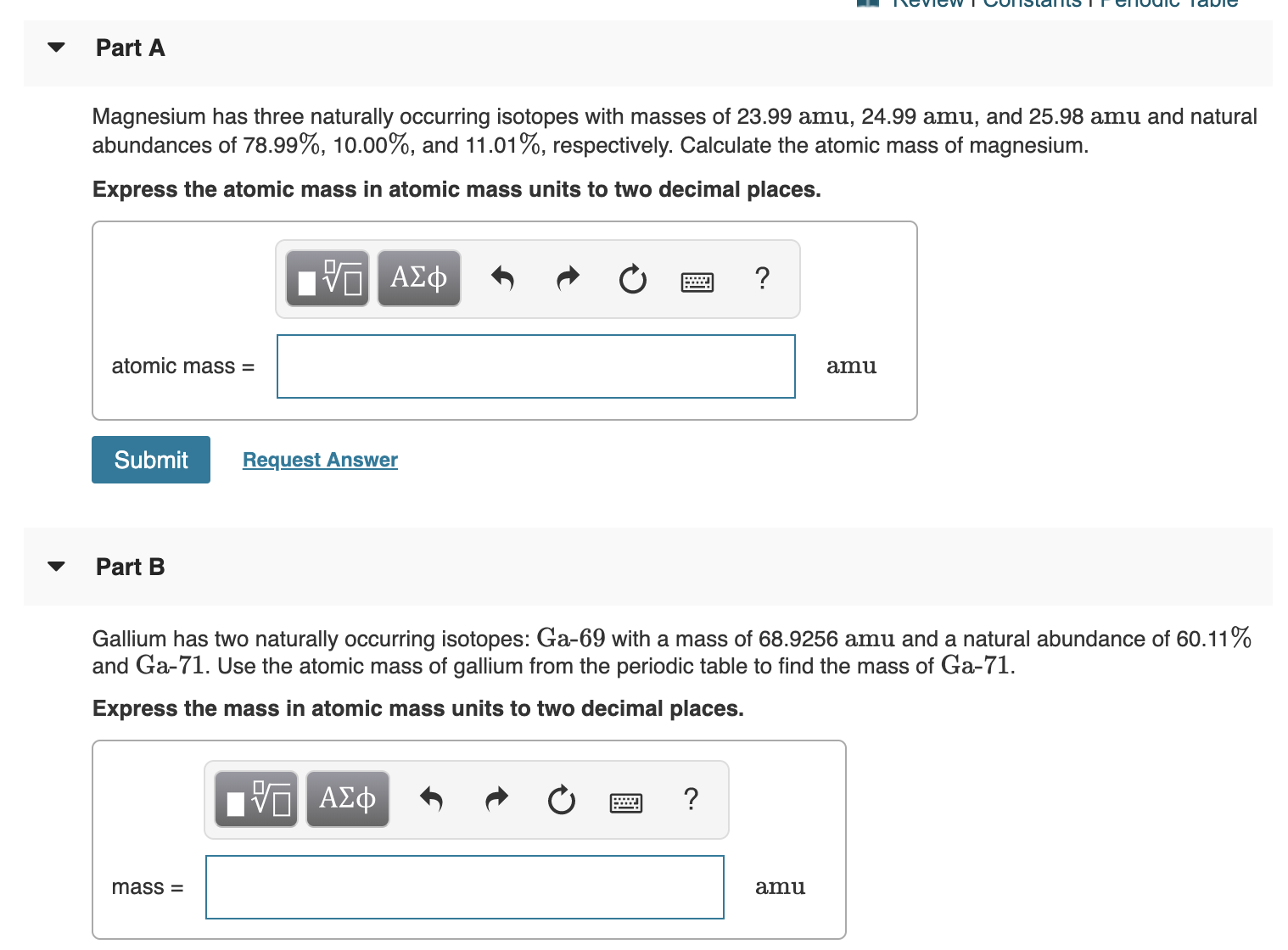
SOLVED:Magnesium has three naturally occurring isotopes: ^24 Mg (23.985 amu) with 78.99% abundance, ^{25} \mathrm{Mg} (24.986 amu) with 10.00% abundance, and a third with 11.01% abundance. Look up the atomic mass of

⚗️Magnesium occurs naturally in only three isotopes. ^24 Mg has an isotopic mass of 23.9850 amu and - Brainly.com

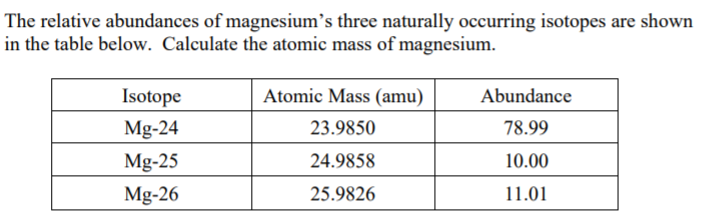
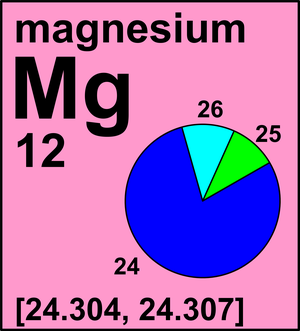
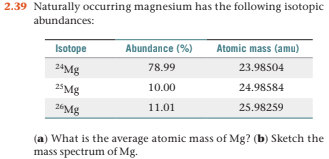
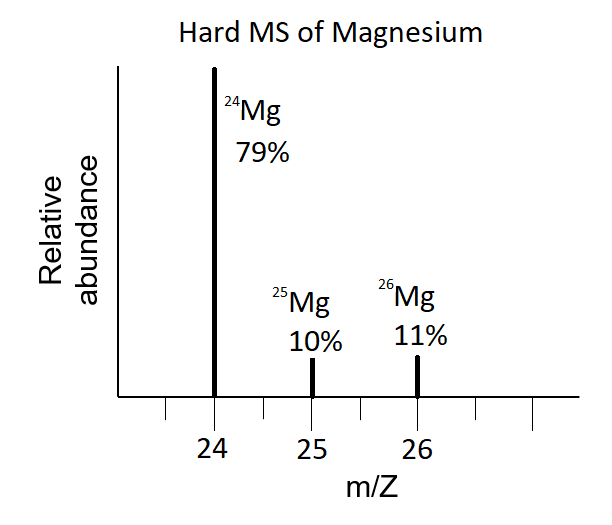
In a periodic table, the averge atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their
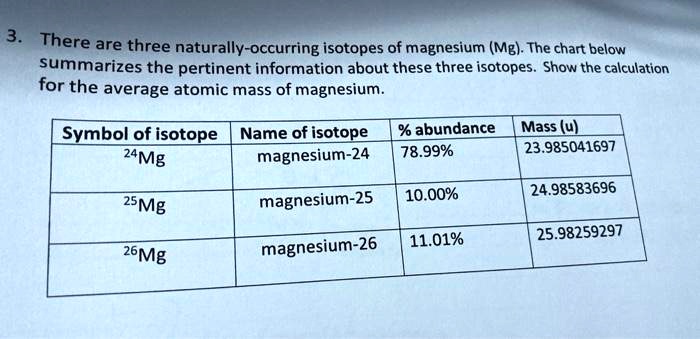
SOLVED: There are three naturally-occurring isotopes of magnesium (Mg) The chart below summarizes the pertinent information about these three isotopes Show the calculation for - the average atomic mass of magnesium. Symbol