SOLVED:The mineral dolomite contains magnesium carbonate. This reacts with hydrochloric acid. MgCO3(s)+2 HCl(aq) →CO2(g)+MgCl2(aq)+H2 O(ℓ) (a) Write the net ionic equation for this reaction and identify the spectator ions. (b) What type

For Your Research. The Four Research Questions 1.What is the chemistry (including an equation) of the process? 2.What are the factors that impact on the. - ppt download
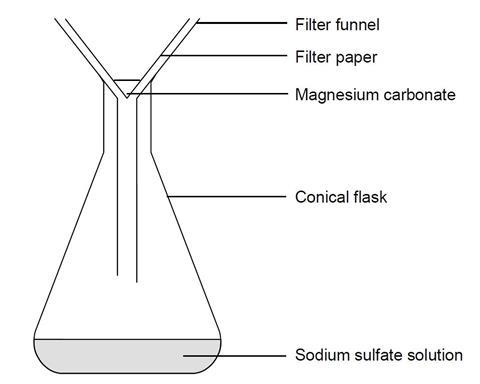
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education
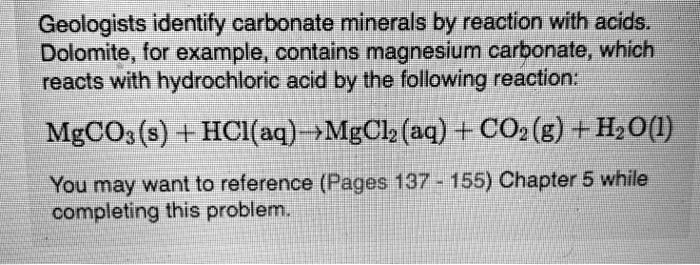
SOLVED: Geologists identify carbonate minerals by reaction with acids Dolomite , for example , contains magnesium carbonate , which reacts with hydrochloric acid by Ihe following reaction: MgCOs (s) + HCl(aq) ,MgClz (

18.4 g of a mixture of calcium carbonate and magnesium carbonate, on heating, gives 4.0 g of magnesium oxide. The volume of CO2 produced at STP in this process is:
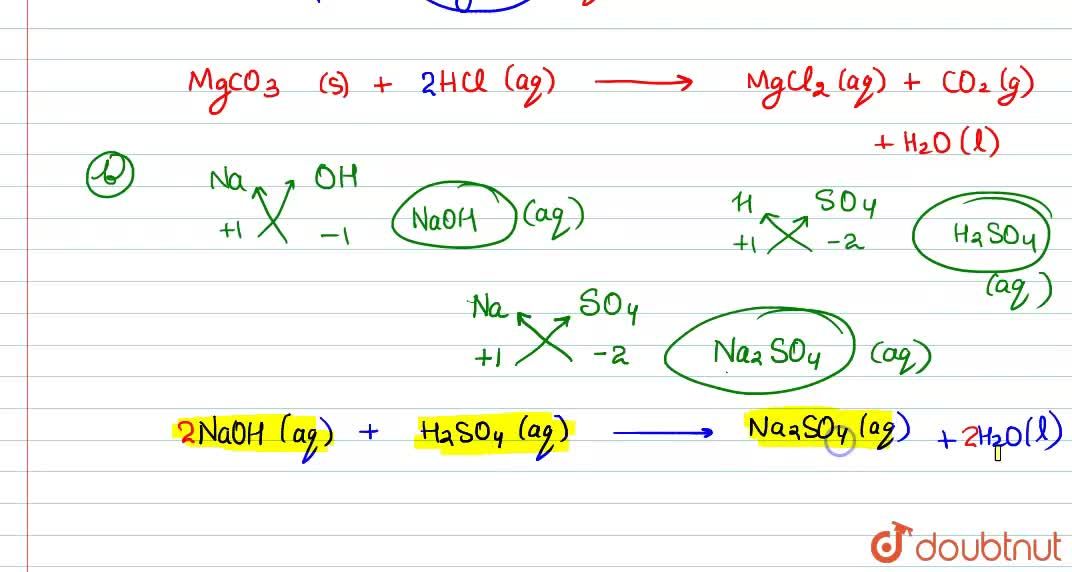
Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrocloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide
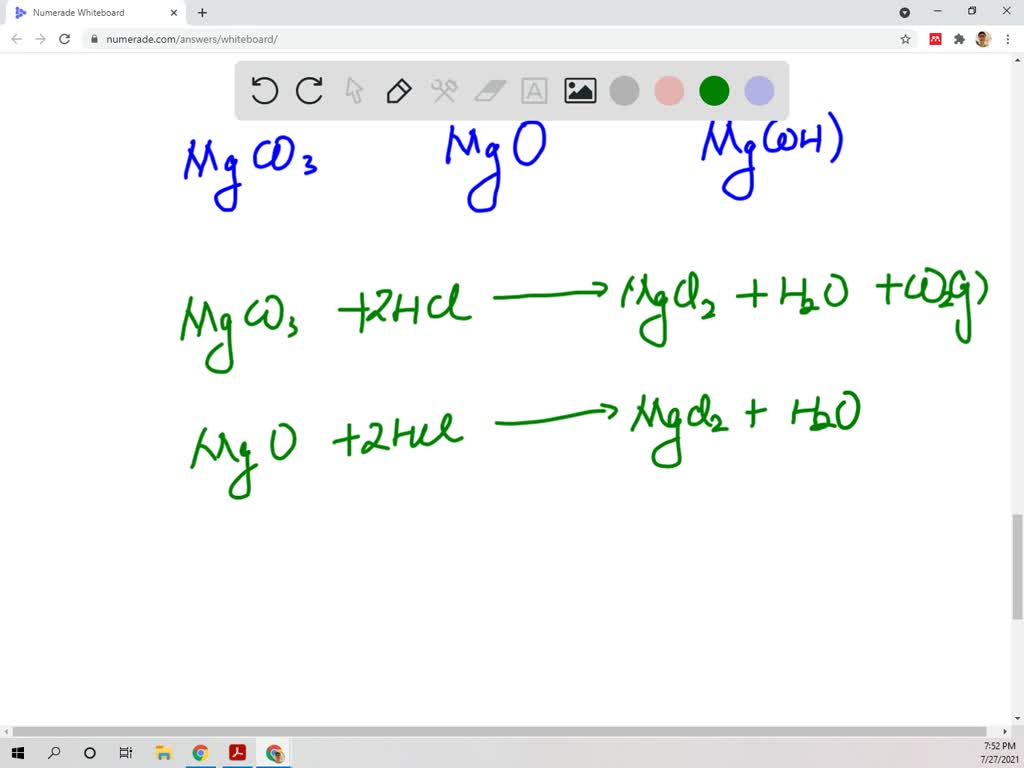
SOLVED: Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a netionic equation for the reaction that occurs

filosoffen.dk - what is metformin 500 mg used for | Commit error. what is the word equation for calcium carbonate and hydrochloric acid congratulate