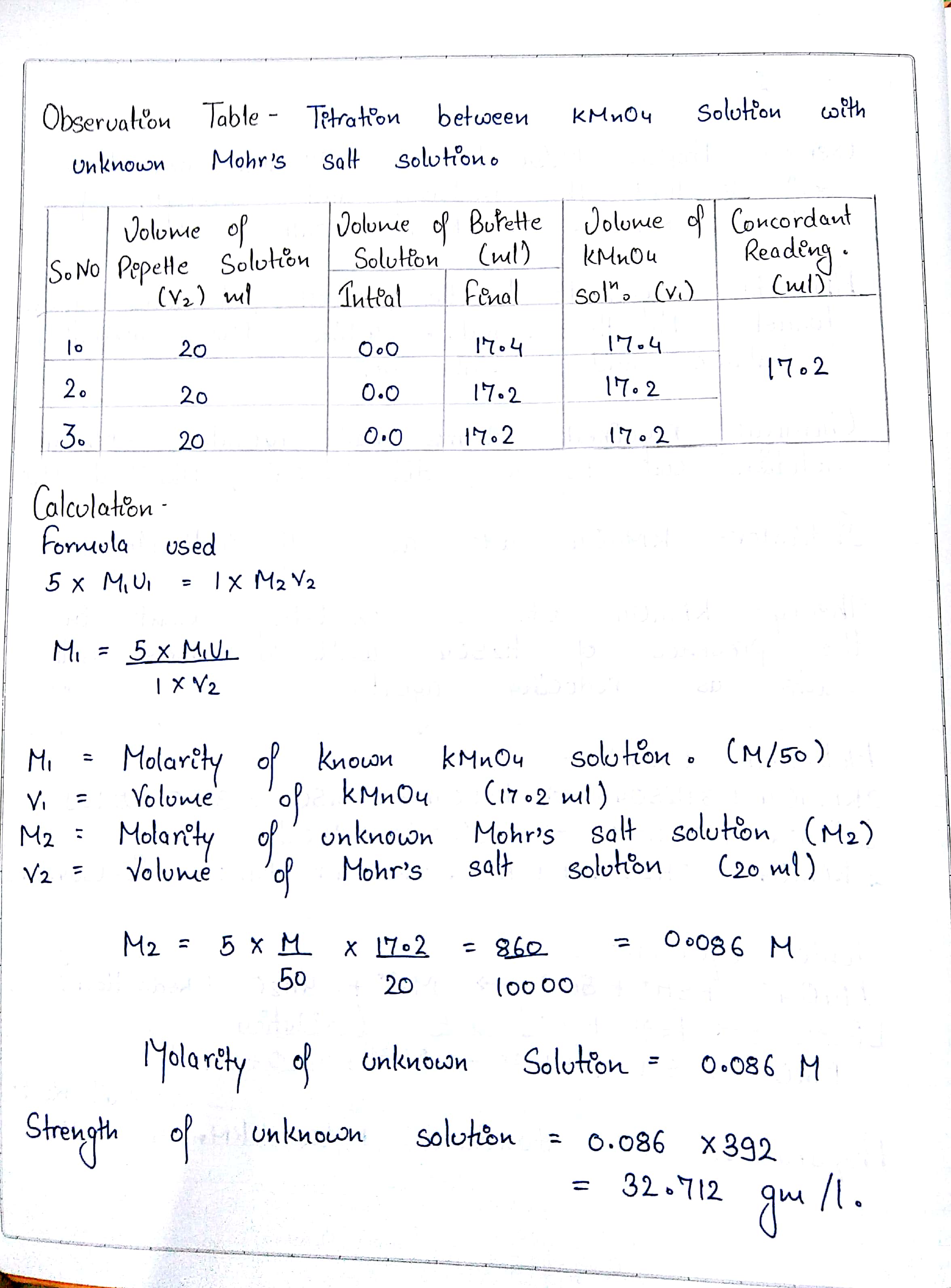
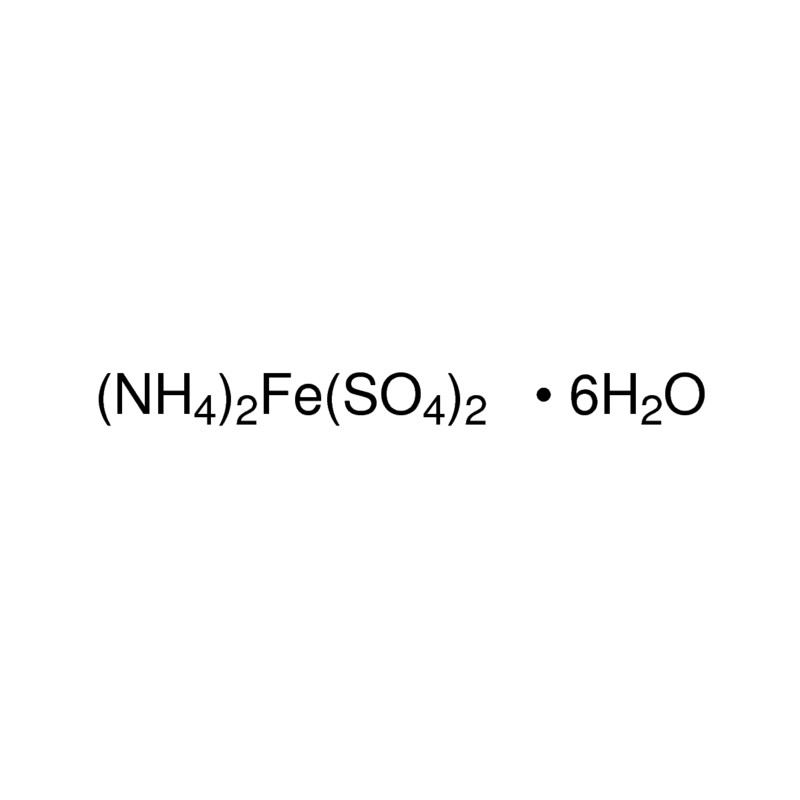When 3.92 g L ^-1 of sample of Mohr's salt reacts completely with 50 mL N/10 KMnO4 solution. The percentage purity of the sample of Mohr's salt is :
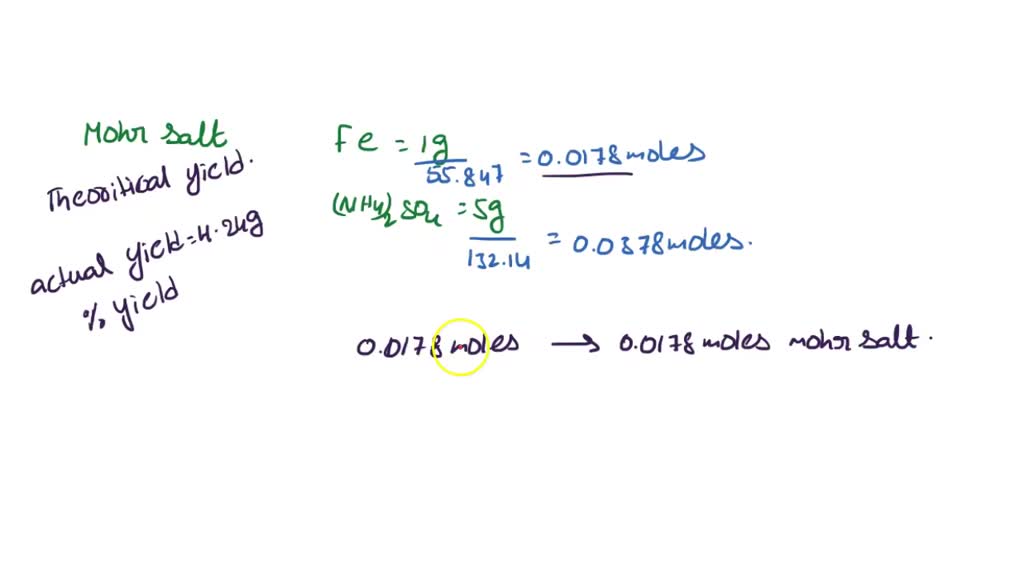
SOLVED: synthesis of Mohr's salt What is the theoretical yield of the preparation when weighing in 1 gram of iron and 5 gram of ammonium sulphate? What is the efficiency with a
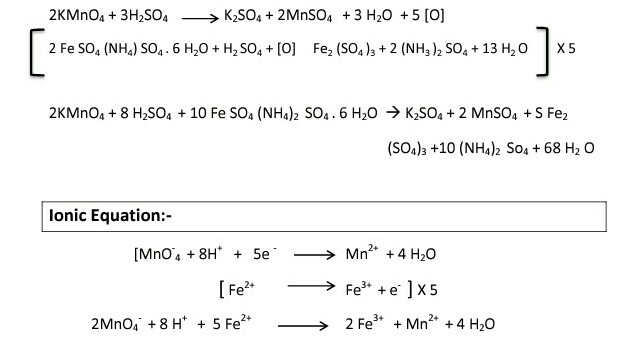
To determine the Molarity of KMnO4 solution by titrating it against a standard solution of Mohr's salt. (M/20 Mohr's salt solution).

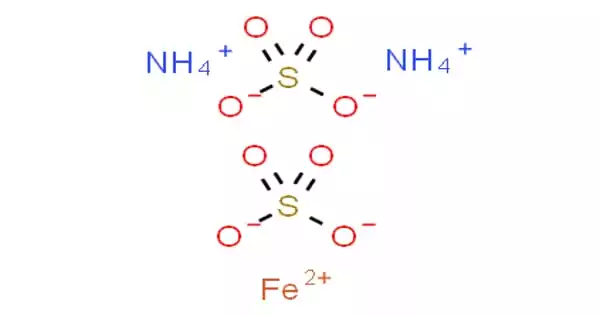
The equivalent weight of Mohr's salt, F eSO 4· N H 42 SO 4· 6 H 2 O is equal to:A. Molar weight of Mohr's saltB. One fourth of the molar weightC.

The molecular formula of Mohr's salt is (NH.),SO.FeS0.6H,O(1) Find the number of atoms of each element.(2) - Brainly.in

Oxidation state of fe in mohr's salt with solution and formula Step wise explanation needed - Chemistry - Redox Reactions - 16519045 | Meritnation.com